past Webinars

ASCO24 Summary: Latest data on ctDNA as a patient selection and early efficacy marker in drug development
sponsored by
Natera

Clinical trials agility: Tips for adapting to study changes and unexpected events
sponsored by
Advarra

Utilizing novel icIEF fractionation and mass spec for the charge characterization of biotherapeutics
sponsored by
Bio-Techne
New ASCO data: Early detection with WGS-based ctDNA tracking up to 1800 variants
sponsored by
Personalis

Case study: Marketing to HCPs using data-based omnichannel segmentation
sponsored by
Definitive Healthcare

Venture capital velocity: What’s driving renewed momentum in biotech
sponsored by
RBC Capital Markets

How real-world research can streamline post-marketing studies in oncology
sponsored by
Flatiron Health

Enabling Adaptive Clinical Trials through Integrated Development and Manufacturing Solutions
sponsored by
Catalent

Enhance your long-term cell & gene therapy success with pDNA manufacturing solutions
sponsored by
Aldevron

Navigating Policy, Funding, CMC and Regulatory Dynamics for Successful Orphan Drug Development
sponsored by
Catalent

Leveraging large-scale mRNA biology data to advance disease understanding
sponsored by
Anima Biotech

3 things every life sciences company should know before leveraging AI to generate real-world evidence
sponsored by
Verana Health

Navigating the clinical AI landscape & charting your path to precision medicine
sponsored by
Mendel AI, Unlearn

7 questions gene therapy developers should ask their CDMO (and what they will ask you!)
sponsored by
Forge Biologics

Untapped potential: Integrating MRD testing to track diffuse large B-cell lymphoma in clinical trials and in the clinic
sponsored by
Adaptive

Post-SABCS summary & year in review: ctDNA in drug development for breast cancer
sponsored by
Natera

Advertising with Endpoints: Our publisher's take on success stories of 2023
organized by
Endpoints News

Advancing Non-Small Cell Lung Cancer drug discovery and treatment with clinicogenomics
sponsored by
Optum

High Lp(a): The unmet need and value of an expansive drug development landscape
sponsored by
Silence Therapeutics

Data-based strategies for successful digital health technology (DHT) clinical trials
sponsored by
Medrio

Oncology case studies: How two life sciences companies applied real-world evidence to drive innovation
sponsored by
Flatiron

Unlocking insight from health data with healthcare-specific AI that thinks like a physician
sponsored by
Mendel

Actionable data: Challenges, solutions and case studies across pharma research
sponsored by
DNAnexus

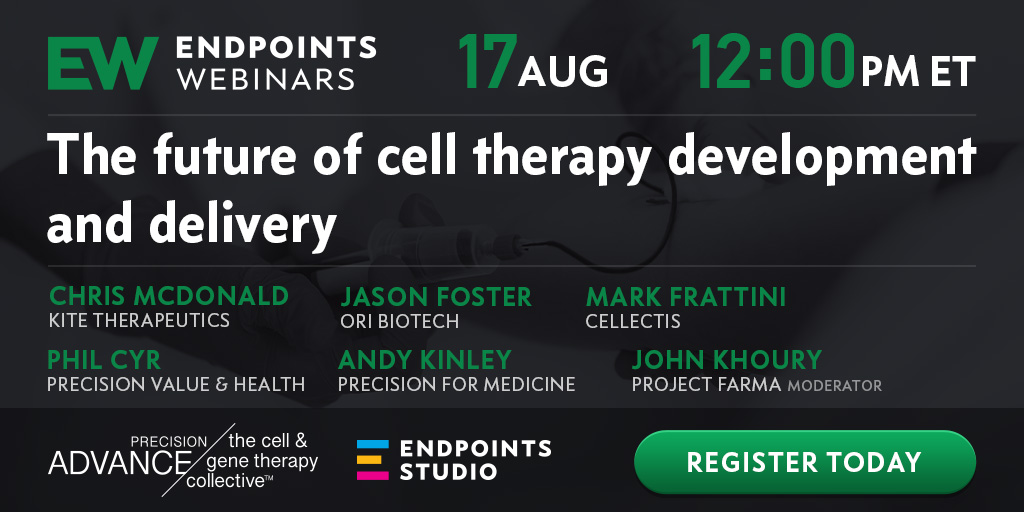
A roadmap to earlier efficacy predictors and drivers in cell therapy development
sponsored by
Adaptive

Harnessing cell power: Targeted protein degraders’ potential in combating disease
sponsored by
Arvinas

Targeted Protein Degraders – Key Strategies to Maximize VC Funding and Clinical Success
sponsored by
Catalent

De-risking clinical development and driving therapeutic innovation: The power of RWD from EHRs
sponsored by
Truveta

Cryo-electron microscopy is revolutionizing rational drug discovery pipelines
sponsored by
Thermo Fisher

How immune repertoire sequencing is addressing unmet needs in multiple sclerosis drug development
sponsored by
Adaptive

Alzheimer’s disease: Using deep human biology learning to guide next-generation treatments
sponsored by
Eisai

Harnessing the power of antigen specific T-cell responses to improve vaccine development
sponsored by
Adaptive

Pharma can target ultra-rare conditions — the next step is financing them
organized by
Endpoints News

Post-ASCO Summary: ctDNA as a patient selection and early efficacy marker in drug development
sponsored by
Natera

Accelerating all phases of the immuno-oncology drug development pipeline via immunosequencing
sponsored by
Adaptive

Transforming clinical research: Scaling real-world data across diseases with expert-led AI
sponsored by
Truveta

The connected lab: maximizing the intersection of science and data
sponsored by
TetraScience Deloitte AWS

How to assess the quality of AI output when structuring unstructured medical data
sponsored by
Mendel AI

Targeted Protein Degraders: Through the Lens of the Innovation, Developability, and CMC Challenges
sponsored by
Catalent

The AI drug-discovery revolution: How NYC is prepared to lead
sponsored by
NYC Economic Development Corporation

When to call it quits: VCs making tough decisions in a tough market
sponsored by
RBC Capital Markets

A novel 3D approach to imaging gene therapy expression and vector biodistribution
sponsored by
Invicro, Emit Imaging

Beyond AAV: Prepare for the next generation of advanced therapy delivery
sponsored by
Center for Breakthrough Medicines

New data: ctDNA as a prognostic and predictive biomarker in early-stage gastrointestinal cancers
sponsored by
Natera

Expediting drug product development and commercialization in global markets with global filing strategies
sponsored by
WuXi AppTec

Endpoints' most popular content this year – an exclusive view for marketers
organized by
Endpoints News

New data: Predict breast cancer treatment response and recurrence risk with ctDNA
sponsored by
Natera

Weird and wonderful case studies: Real-world solutions for unexpected bioanalytical challenges
sponsored by
Agilex Biolabs

Patient need for novel therapies in ROS1 fusion-positive non-small cell lung cancer
sponsored by
AnHeart Therapeutics

Navigating industry challenges to drive sustainable Plasmid DNA strategies
sponsored by
Charles River

Lessons learned in bringing novel options for rare liver diseases to patients and families
sponsored by
Albireo Pharma

The Biotech landscape: Market trends, priorities, predictions, and pathways to FDA approval
sponsored by
Novotech

Advanced dosage forms: Tackling drug delivery challenges & addressing patient needs
sponsored by
Catalent

The evaluation of pan-cancer and oncoReveal™ CDx panels to support localized NGS testing
sponsored by
Pillar Biosciences

A journey without a map: lessons in building an oncology powerhouse where innovation thrives
sponsored by
Astellas

Changing the paradigm for colorectal cancer: Clinical practice & drug development
sponsored by
Natera

A crisis in rare disease: How the current investment landscape is stifling rare disease development
sponsored by
Precision Medicine

Integrated clinical development and supply: Breaking down the early-development journey of a small molecule
sponsored by
Catalent

Identify and quantify point mutations in circulating tumour DNA: The ddPCR approach™
sponsored by
Agilex Biolabs

New data: How ctDNA is evolving oncology patient management and drug development
sponsored by
Natera

Accelerating first-in-class and best-in-class programs using a large-scale digital chemistry strategy
sponsored by
Schrödinger

Applying machine learning to increase clinical trial efficiency: a regulatory journey
sponsored by
Unlearn

R&D changemakers: Building Resilience’s digital infrastructure for a modern R&D team
sponsored by
Benchling

Diagnostics from digital pathology: an AI solution to accelerate precision medicine
sponsored by
OWKIN

Navigating your biopharma drug candidate through early and mid-stage clinical trials
sponsored by
Catalent

Using external data to accelerate randomized controlled trials without introducing bias
sponsored by
Unlearn

mHealth innovation: identifying the best patient-centered technology for your trial needs
sponsored by
Parexel

Changing the game: an examination of cutting-edge novel oncology screening tools
sponsored by
Charles River

A new class of startups is scouting for deals - on both sides of the Pacific
organized by
Endpoints News

Can changing one step change everything? Replacing your polishing step with a single-use solution
sponsored by
3M

Non-clinical and clinical pathways for rapid vaccine development in Australia
sponsored by
Agilex Biolabs

The neurosciences comeback: After a lengthy chill, Big Pharma is warming back up to neuro
organized by
Endpoints News

Essential strategies for gene therapy - from assay development to CDx commercialization
sponsored by
Precision Advance

Innovation in drug product manufacturing: how do we bring new therapies to patients faster?
sponsored by
Cytiva

Life science SPACs – the future of IPOs/crossover investing? A look at 2021/2022
sponsored by
Back Bay Life Science Advisors

Reinventing Harvest Technology: Single-stage chromatography clarification for recombinant protein therapeutic manufacturing
sponsored by
3M

Are we ready for the medicines of tomorrow? Creating the infrastructure for the era of genomic medicine
sponsored by
Sangamo Therapeutics

Plasmid DNA Insights: Expanded focus on CMC in gene & cell therapy development
sponsored by
Aldevron

Transformative approaches to clinical development: Scaling adoption across the industry
sponsored by
Deloitte

Hot topics in Immunoassay Bioanalysis - navigating the immunogenicity and biomarker requirements for clinical trials
sponsored by
Agilex Biolabs

The Great Debate: Internal vs. external manufacturing for advanced therapies
sponsored by
Precision Medicine Group

Predict placebo response using patient psychology to increase trial power
sponsored by
Tools4Patient

Can sponsors collect data in Expanded Access (EAPs) and Named Patient Programs (NPPs)?
sponsored by
WEP Clinical

Clinical trial diversity in the Covid-19 era — are we seeing a paradigm shift?
organized by
Endpoints News

Flattening the 'valley of death:' New tools in the hunt for better preclinical R&D
organized by
Endpoints News

Patient-First R&D: Why the patient experience should be at the center of clinical trial design
sponsored by
AbbVie

Enabling multidimensional tumor immunogenomics for advancing biomarker discovery
sponsored by
Personalis

Breakthrough Technologies to Accelerate Biologic Drug Discovery, Cell Line Development and Biomanufacturability
sponsored by
AbSci

How the World’s First Biolabs Client Portal GALEXI™ Delivers Real-Time Study Management for Biotechs
sponsored by
Agilex Biolabs

Optimizing Direct-to-Patient Clinical Supply for Decentralized Clinical Trials
sponsored by
Catalent

Innovation in Clinical Research: AI-based Drug Development Tools and the Regulatory Landscape
sponsored by
Unlearn.AI

R&D in an age of Covid-19: Tackling the key issues around starting, or restarting an early-stage study
sponsored by
Egnyte

New Platform for Target-to-Hit: From Fragment Screen to Structure-based Drug Discovery
sponsored by
WuXi AppTec

Why Australia is the World’s Leading Early Phase Destination – Rapid Start-up, No IND Required, and a Government-Backed Refund on Almost Half of all Trial Costs
sponsored by
Agilex Biolabs

The Transformation of Clinical Trial Design and Operations: A Panel Discussion on COVID-19 as a Catalyst for Decentralized Trials
sponsored by
PPD

When cell and gene therapies surge to market, how resilient will your cell sourcing infrastructure be?
sponsored by
Be The Match

The Australian Early Phase Biosimilars Clinical Trial Landscape - How Agilex Biolabs and Nucleus Network Work Together
sponsored by
Agilex Biolabs

A Clinical Investigator’s Perspective: Novel Oncology Therapy During & Beyond the COVID Pandemic
sponsored by
Worldwide Clinical Trials

Improving Clinical Trial Quality with Best Practices for E-Clinical Technology
sponsored by
Medidata

ctDNA as a biomarker for monitoring treatment response and molecular recurrence in early-stage and metastatic cancers
sponsored by
Natera